E Coli Protein to rRNA Attachment Site Maps
Background
The Ribosome of the E Coli is one of the most well understood larger organelles. The Noller Lab has produced beautiful images that document what we’ve found with crystallography.
I recommend going to their web page for a deep visualization of how it works:
And here’s a fun gif animation…
By Bensaccount at en.wikipedia, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=8287100
See where the mRNA enters and leaves the small subunit and where the amino acid chain, the final protein leaves the top of the large subunit. It switches at some point to exporting that protein.
Here’s the rRNA secondary structure diagram the Noller lab produced for 16s - the rRNA for the small subunit (30s):
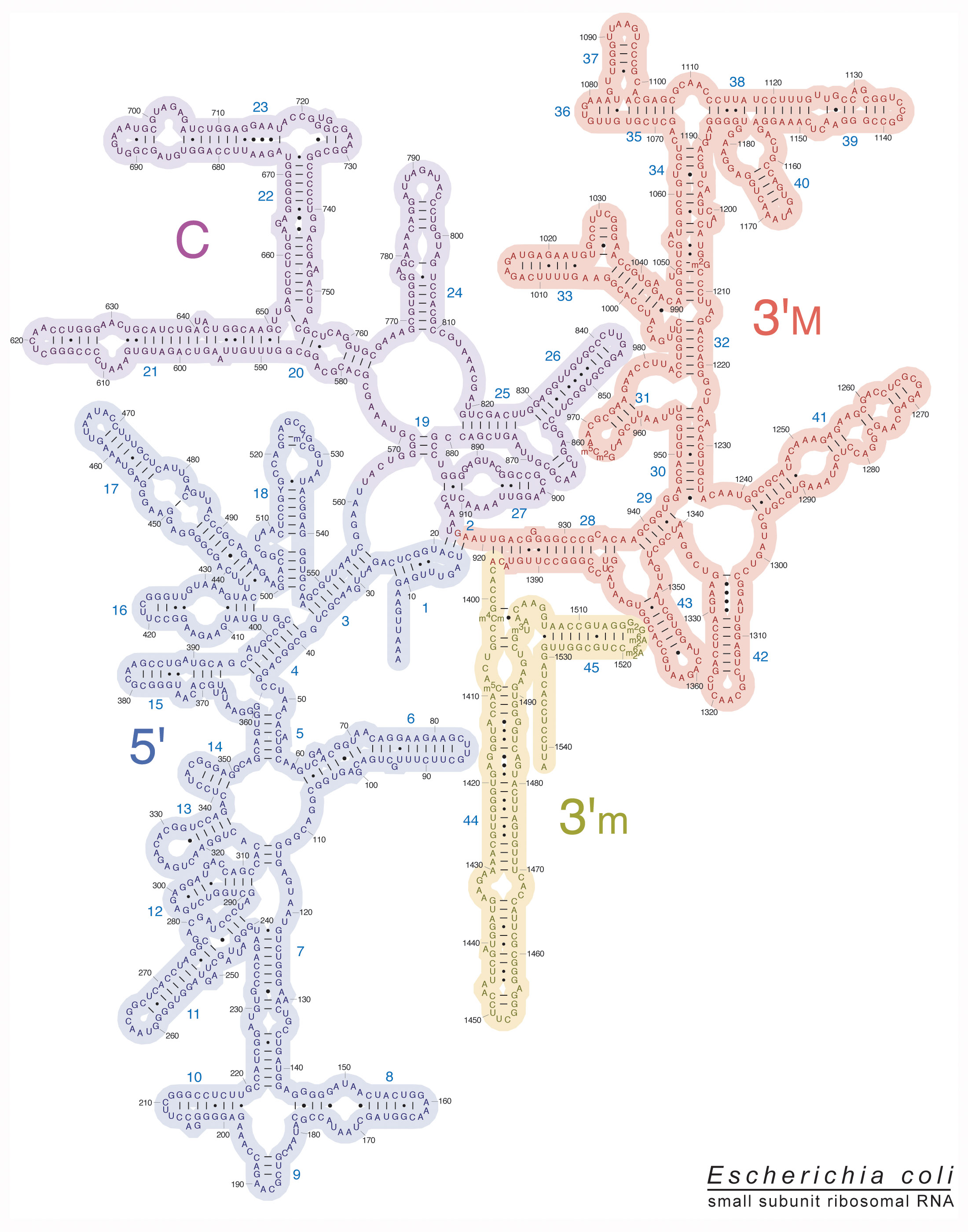
The pattern is created based on the natural nucleotide bonding patterns - stems and loops. The actual layout is curated to match known traits of the physical structure. So it’s known what’s a stem, what’s a loop, and where it is found. The artists who created these maps helped crystalize that into these images - from crystallograpy.
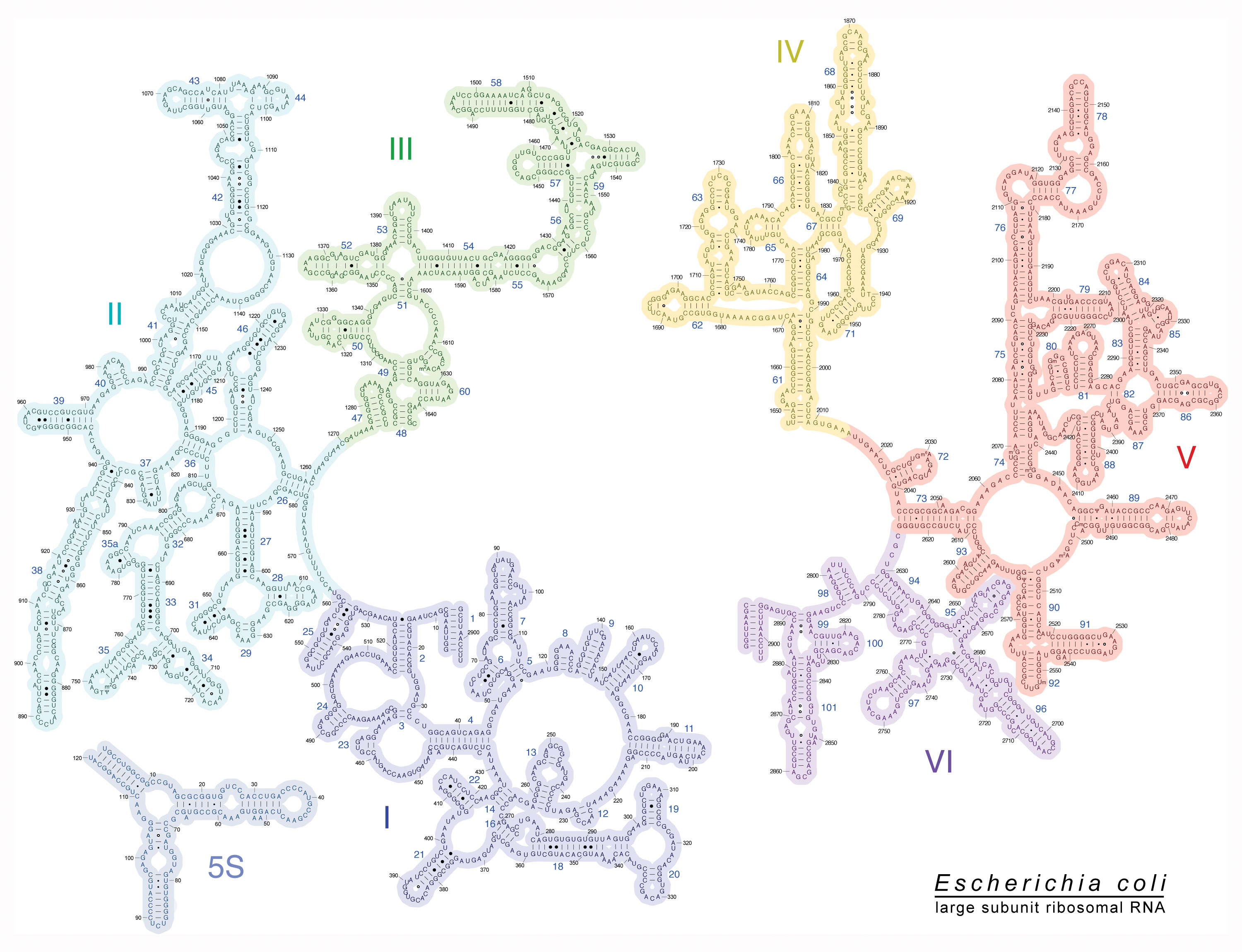
Here’s the rRNA, 23s, for the large subunit (50s):
Matching DNA to rRNA
By simply matching the DNA codon sequences for Ribosomal proteins, I found structural matches for ten attachment sites in E Coli, involving six Ribosome proteins: rplD, rplB, rpsD, rpsH, rpsE, rplC.
Then I found three more sites in the human rRNA 18s with the S4 encoding genes: rps4x and rpl4 genes matching sites similar to E Coli’s S4, but each was slightly longer.
For rps4x 1-13 and rpl4-11, they were both on similar shaped sites as E Coli. I checked rpl4@283 because I saw in Alpha Fold that it was a ribbon section, near rpl4 1-11. I found it right nearby - right next to rpl4’s 1-11 site in the secondary structure diagram. The patterns are subtle, flowing but the back-and-forth twisting patterns, long sequences of nearly perfect matches.
Some of the patterns look “improbably stretched” in the secondary structure diagram until you see that they are also at structural regions - the top of a loop, or the neck of a stem, one of those weird bumps on a loop. These are where a flowing bond makes sense structurally. These may be multiple simultaneous attachment points for flowing structures, alternate bonding sites based on fluid flow patterns, or redundant layers - built in so this site does not slip. Could it be a coincidence?
- Two organisms, eleven genes - all the ones where I had confidence I knew where to look, found where expected. All sites with consecutive or nearly consecutive perfect matches between the protein’s codon and the rRNA. See the table below for specific codon index to rRNA letter index matches.
This is data that is widely available, well studied but no one thought to put it together. And yet there is such a high degree of correlation, clear structural patterns, that look designed and match what we know about the ribosome’s form and function.
Multiple proteins, sharing the same attachment sites, for redundancy, flowing assembly? Or maybe the rRNA has enough bonding energy for them all to hold them in a shape? It does keep getting recharged with energy from the ATP charged tRNA that continually connect up.
From the structural pictures, it feels like these are flowing bonds, adjusting shapes, backups for important attachments.
E Coli 23s Attachment Sites
The patterns are so consistent and structurally logical that they suggest an organizational principle we’ve been missing. Here’s what the binding sites look like one-by-one so you can see how the consecutive proteins map to the sites:
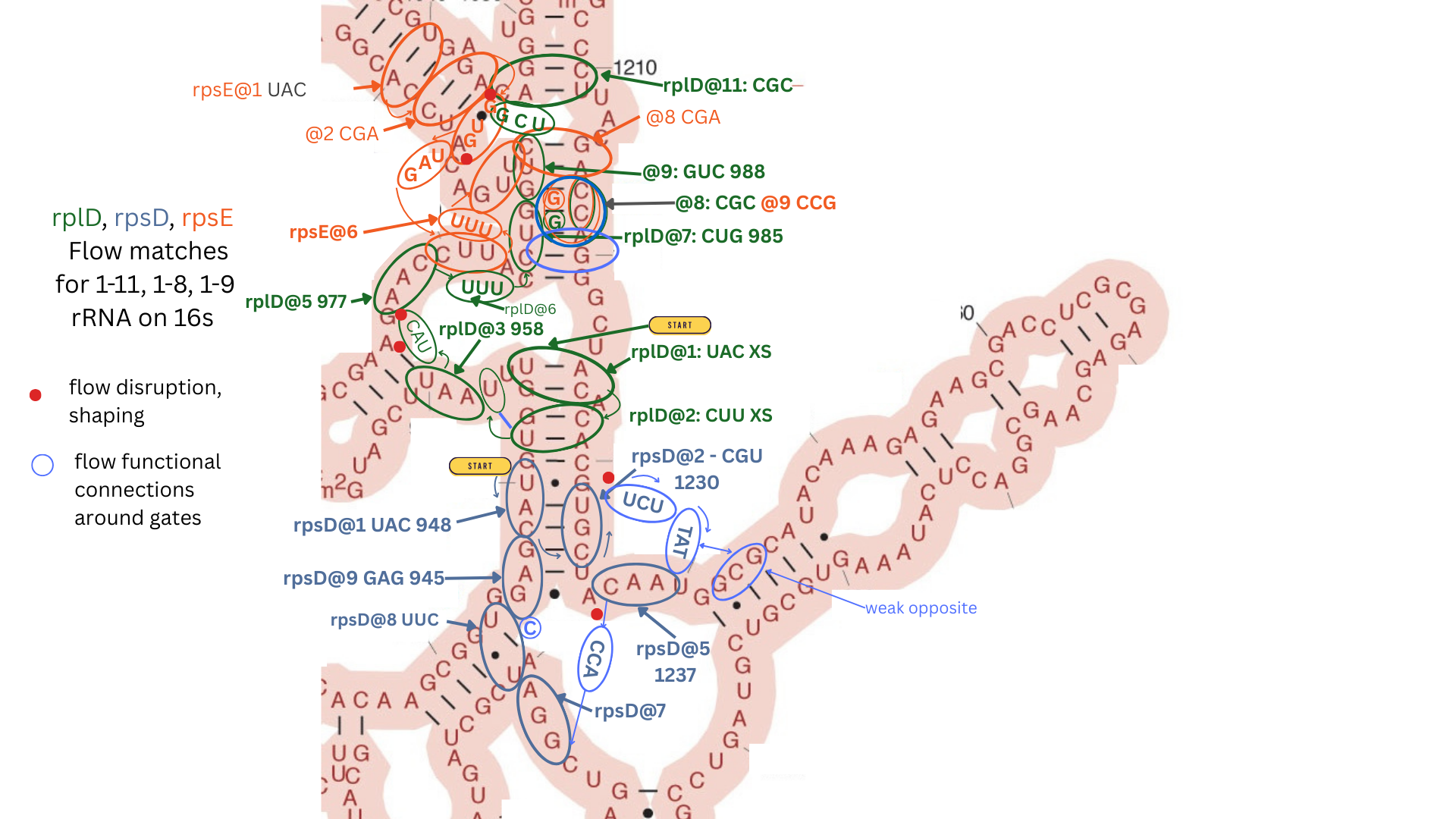
E Coli 16s Attachment Sites
H. Sapiens 18s - rps4X
H. Sapiens 18s - rp4L
Observations
These proteins are all found roughly where they are expected from crystallography. The patterns jump out when you have the sequences and are looking in the right place. When I’m looking in the wrong place, I can stare it for hours and not find anything.
The attachment sites correspond to regions of similar shapes when comparing them to Alpha Fold’s images: all relatively flat spots, strands, ribbons, not helices.
Each attachment site has a clear entrance and exit that makes sense structurally.
Attachment sites all are structural: on stems, loops, crossing small open sections (ponds) and between stems (bays).
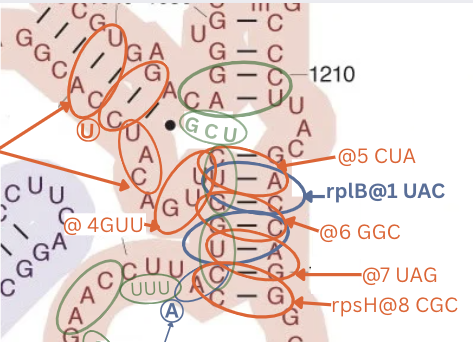
- Here’s the longest matching sequence - rpsH on 16s with 22 codons in a row with only one codon out of sequence, down one stem around a loop and down another using an opposite pattern to rplB on the same stem.
- rplB has the most beautiful sequence: two bonds on a long stem. In the middle of the stem the rRNA has: G - U and U - G - the weak opposites next to each other in symmetry, creating a more flexible part. Right in the middle of this long stem. On top of that comes the protein next has CCA - opposites to G and G but A and U are attracting.
This creates a crazy flowing bond/repel action right where this “bendy” section of the long stem is. Follow that up with two more three letter matches, then cross a big loop and another three letter match. Further, rplB is on 16s, interacting on a similar stem with rplD. This create a chain of fluid reactions, coordinated by the fluid energy exchange of the tRNA.
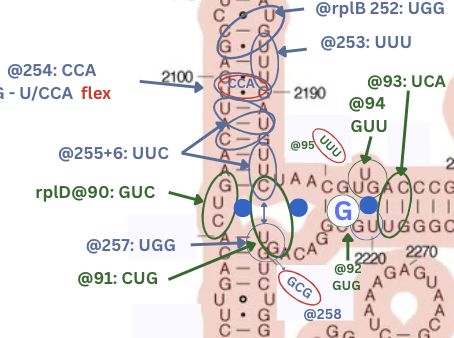
- Sites are shared by multiple proteins and for the two proteins rplD and rplB that were found on both 23s and 16s, the interaction patterns match on both sites. In particular on 23s, rplD crosses an open pond:
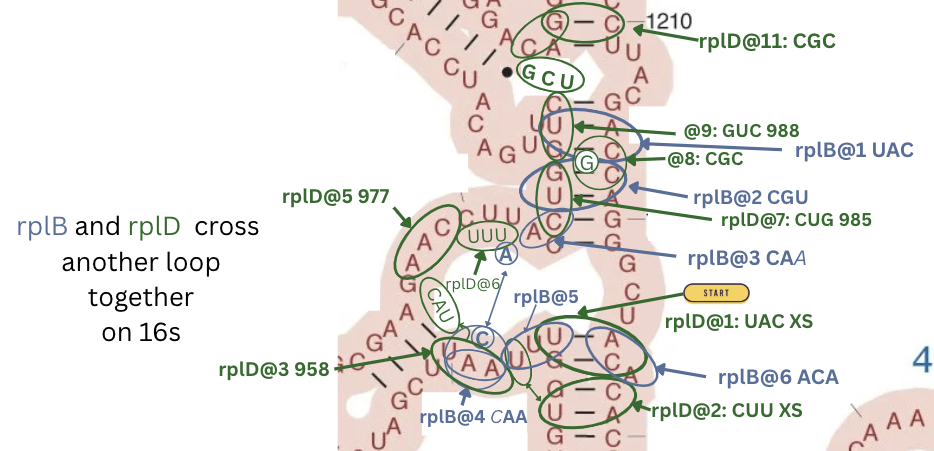
and here again on 16s.
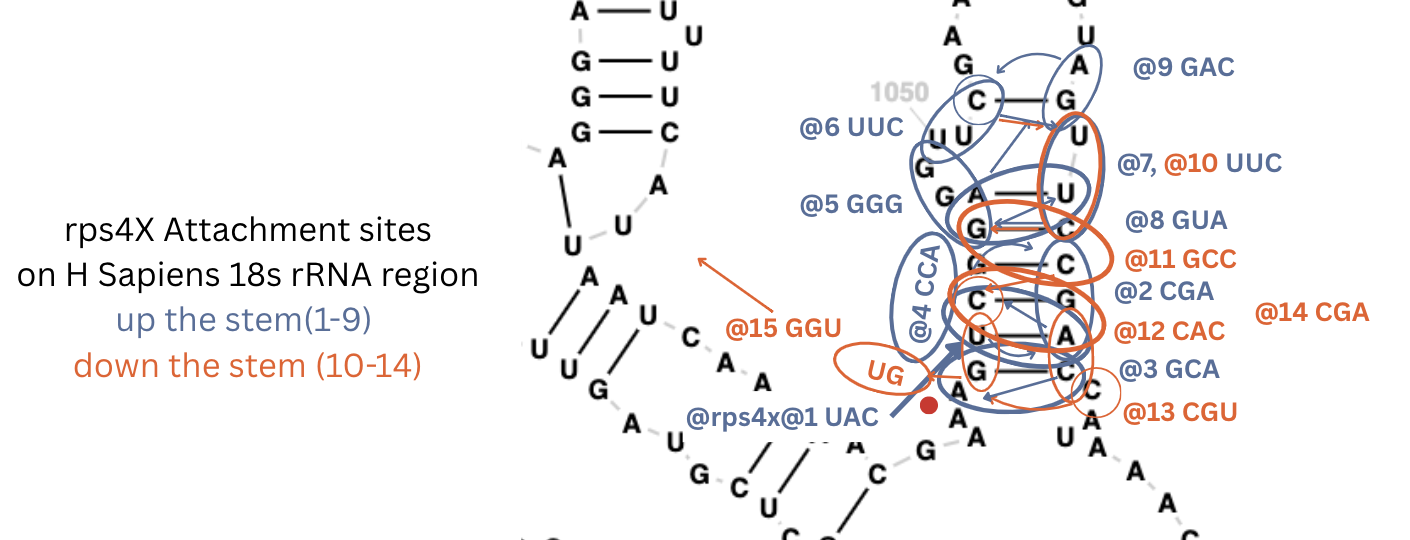
For the more complex human genome, so far I only looked for one gene - the one for S4 - rps4x and found it in about an hour on 18s. It’s the most complex attachment site yet with 9 codons going up the stem and five coming back down.
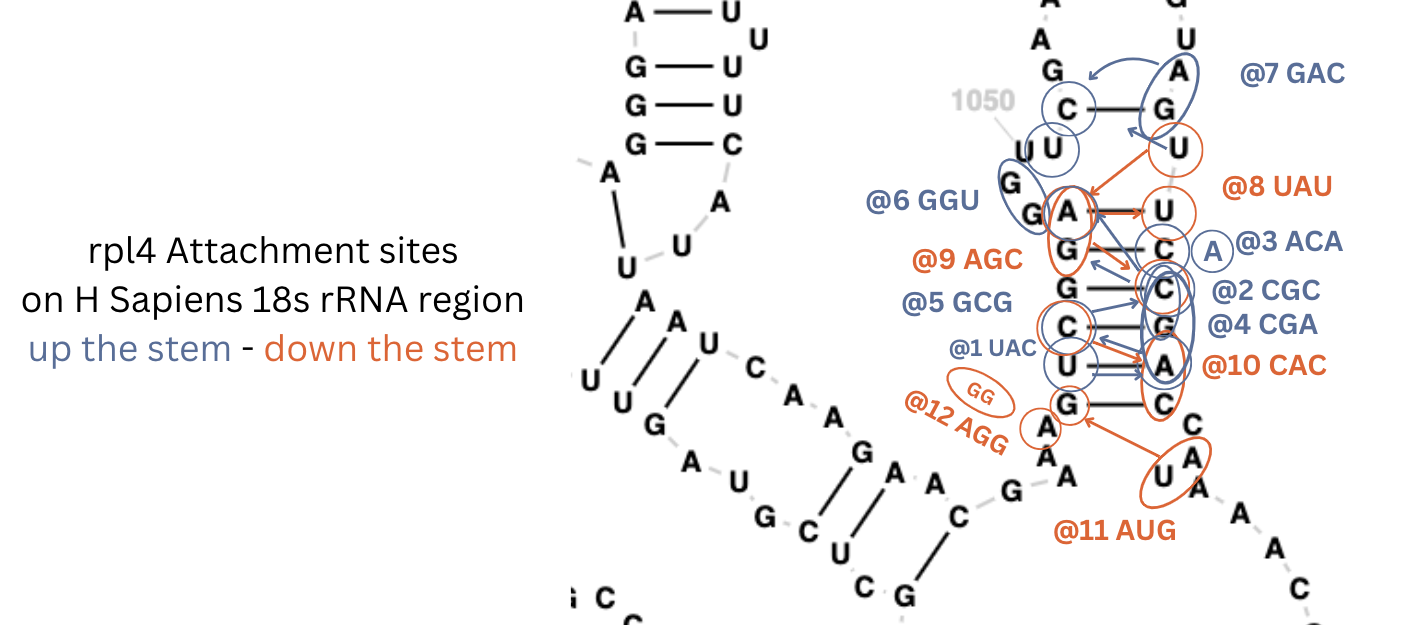
and similar detail for rpl4 1-11.
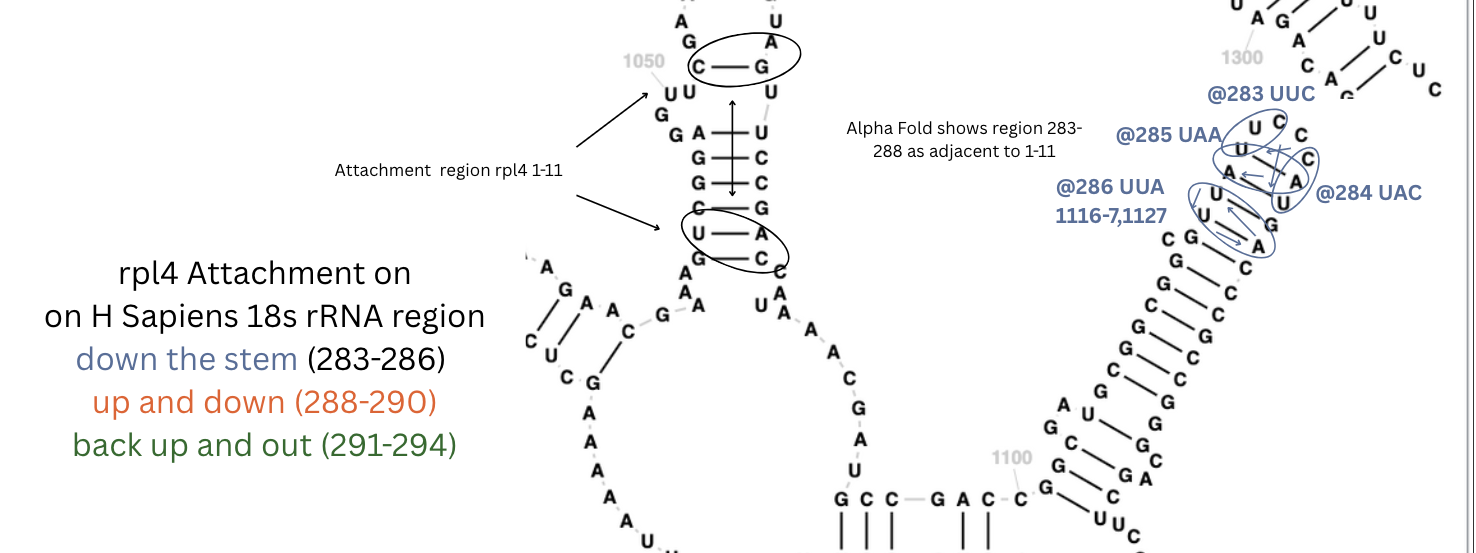
and even more complex for rpl4 283-293. As predicted by alpha fold, next to the 1-11 site.
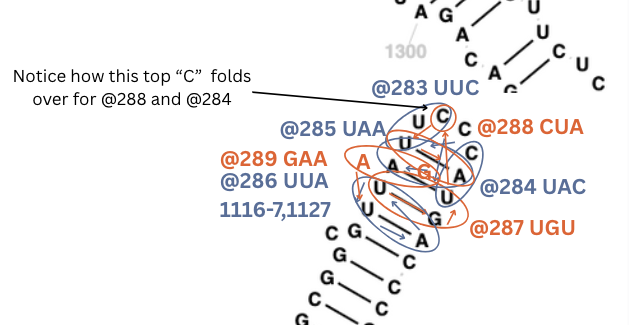
The top ‘C’ folds over into that small pond for two of these bonds.
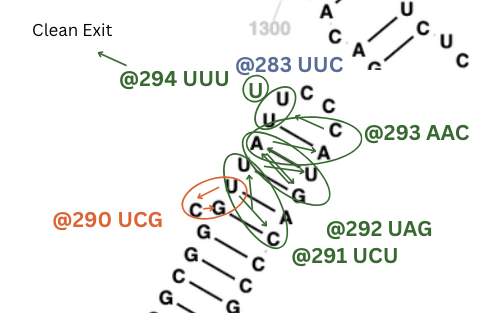
Three layers of overlapping patterns going up and down the same stem.
All of the bonds are twisting patterns practically and the exit is a perfect UUU right in the center of a loop with complementary patterns.
Note on Notation
For convenience purposes, only the rRNA’s pattern is shown, i.e. the DNA’s codon’s anti-codon is shown. For each match, consecutive letters in the DNA match consecutive letters in the rRNA. Matches go in either direction, zig-zag, etc. but the order is preserved like how the amino-acids will be organized in the protein chain.
Nucleotide’s have these opposite patterns between DNA and RNA:
DNA <=> RNA
G <=> C
C <=> G
A => U
T => A
T ~= USo rplD@1's first codon is ATG but the table shows the mirror UAC that will match directly to rRNA’s UAC at these letter positions 955,1224,1223.
Flow
Flow type of 888 means that each of the three DNA letters in the codon matches it’s opposite in the rRNA.
A big X indicates the perfect conflict where: G would meet C, or A would meet U. These are exit patterns. And to be clear, this is where the letter in the DNA Codon matches the rRNA letter - same spinning signatures repel other same-spinning signatures.
A small x for a letter indicates the non-perfect conflict - e.g. G repels U but less so than C, and A repels C but less so than U.
A small w for a letter indicates the weak match - e.g. G is friendly with A, U is friendly with C.
Stem Patterns
Stem patterns are numbered from the perspective of the start codon:
*1 . 2 2 . *1
3 . 4 4 . 3
5 . 6 6 . 5
5 . 6 6 . 5
3 . 4 4 . 3
*1 . 2 2 . *1The Type column is an attempt to keep track of direction but it goes back and forth so that’s a work in progress.
E Coli 16s/23s Mapping Table
| Protein@Codon | rRNA Indices | Type(WIP) | Match Pattern | rRNA Region | rRNA code | Flow | Partners |
|---|---|---|---|---|---|---|---|
| rplD@1 | 955,1224,1223 | flipped | stem-124 | 16s 3’M | UAC | 888 | |
| rplD@2 | 1228,952,967 | stem-bay-cross | 16s 3’M | CUU | 888 | ||
| rplD@3 | 958,959,960 | normal | loop | 16s 3’M | AAU | 888 | |
| rplD@4 | 975,976 | normal | pond-cross | 16s 3’M | CAU/AG | xx- | |
| rplD@5 | 977,978,979 | normal | loop | 16s 3’M | AAC | 888 | |
| rplD@6 | 980,981,982 | normal | loop | 16s 3’M | UUU/CUU | w88 | rpsE@6 |
| rplD@7 | 985,986,987 | normal | stem-135 | 16s 3’M | CUG | 888 | |
| rplD@8 | 1218,1217 | normal | stem-13 | 16s 3’M | CGC/CC | 8x8 | rpsH@6,… |
| rplD@9 | 988,989,990 | normal | stem-135 | 16s 3’M | GUC | 888 | rpsH@5,… |
| rplD@10 | pond-cross | 16s 3’M | GCU | — | |||
| rplD@11 | 1045,1046,1210 | loop-stem-jump | 16s 3’M | CGC | 888 | ||
| rplD@12 | 1211,1209 | exit | bulge-exit | 16s 3’M | GAC | XXX | |
| ————— | |||||||
| rplD@63 | 2076,2075,2074 | flipped | loop-stem | 23s V | UUU | 888 | |
| rplD@64 | 2073,2072,2443 | flipped | stem-134 | 23s V | CCG | 888 | |
| rplD@65 | 2441,2439,2069 | flipped | stem-loop-cross | 23s V | UUG | 888 | |
| rplD@66 | 2442,2443,2444 | normal | stem-135 | 23s V | CCG | 888 | |
| rplD@67 | 2445,2065 | stem-loop-1x | 23s V | GCA | 88x | ||
| rplD@68 | 2066,2067,2442 | normal | stem-loop-1x | 23s V | CGC | 888 | |
| rplD@69 | exit | bay-exit | 23s V | CGC | XXw | ||
| rplD@90 | 2091,2092,2093 | normal | loop-stem | 23s V | GUC | 888 | |
| rplD@90 | 2091,2092,2093 | normal | loop-stem | 23s V | GUC | 888 | |
| rplD@91 | 2196,2229,2228 | pond-cross | 23s V | CUG | 888 | rplB@257,rplB@256 | |
| rplD@92 | 2221,2220 | pond-cross-1x | 23s V | GUG | -88 | ||
| rplD@93 | 2219,2206,2205 | stem-142 | 23s V | UCA | 888 | ||
| rplD@94 | 2204,2203,2202 | stem-bump | 23s V | GUU | 888 | ||
| rplD@95 | exit | bay-exit | 23s V | UUU | xwX | ||
| ————— | |||||||
| rplB@1 | 991,1216,1217 | normal | stem-124 | 16s 3’M | UAC | 888 | |
| rplB@2 | 1218,987,986 | normal | stem-124 | 16s 3’M | CGU | 888 | |
| rplB@3 | 988,990 | normal | stem-loop-1x | 16s 3’M | CAA | 88- | |
| rplB@4 | 959,958 | normal | loop-receive-1x | 16s 3’M | CAA | -88 | |
| rplB@5 | 957,956,955 | normal | loop | 16s 3’M | UUU | 888 | |
| rplB@6 | 1225,1226,1227 | normal | stem-with-bump | 16s 3’M | ACA | 888 | |
| ————— | |||||||
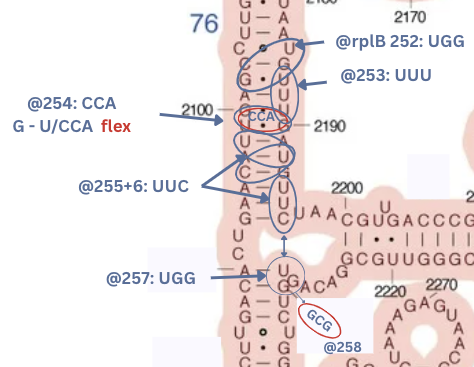
| rplB@252 | 2185,2186,2112 | normal | stem-136 | 23s V | UGG | 888 | |
| rplB@253 | 2189,2188,2187 | normal | stem-124 | 23s V | UUU | 888 | |
| rplB@254 | 2100,2099,2190 | mid-stem-flex | 23s V | CCA/GUUG | XwwW | ||
| rplB@255 | 2098,2192,2096 | flipped | stem-145 | 23s V | UUC | 888 | |
| rplB@256 | 2194,2195,2196 | normal | stem-135 | 23s V | UUC | 888 | rplD@91 |
| rplB@257 | 2229,2228,2230 | loop-stem | 23s V | UGG | 888 | rplD@91 | |
| rplB@258 | exit | pond-exit | 23s V | GCA/UA | xw– | ||
| ————— | |||||||
| rpsD@1 | 948,947,946 | flipped | stem-135 | 16s 3’M | UAC | 888 | |
| rpsD@2 | 1230,1231,1232 | normal | stem-135 | 16s 3’M | UCG | 888 | |
| rpsD@3 | 1229 | bay-cross | 16s 3’M | UCU | — | ||
| rpsD@4 | 1244,1243,1242 | weak-opposite | 16s 3’M | TAT/GCG | www | ||
| rpsD@5 | 1237,1238,1239 | normal | loop | 16s 3’M | CAA | 888 | |
| rpsD@6 | 1240 | pond-cross | 16s 3’M | CCA/A | x– | ||
| rpsD@7 | 1339,1338,1337 | flipped | loop | 16s 3’M | GGA | 888 | |
| rpsD@8 | 1340,1341,943 | flipped | neck-weak-opposite | 16s 3’M | UUC/A | 8x8 | |
| rpsD@9 | 945,946,947 | normal | loop-stem | 16s 3’M | GAG | 888 | |
| rpsD@10 | 948 | exit | mid-stem-exit | 16s 3’M | UUC/G | x– | |
| ————— | |||||||
| rpsH@1 | 1040,1000,999 | flipped | stem-124 | 16s 3’M | UAC | 888 | rpsE@1 |
| rpsH@2 | 998,1043 | stem-12 | 16s 3’M | UCG | -88 | rpsE@2 | |
| rpsH@3 | 997,996,995 | flipped | loop | 16s 3’M | UAC | 888 | |
| rpsH@4 | 993,992,991 | flipped | loop | 16s 3’M | GUU | 888 | |
| rpsH@5 | 990,989,1216 | flipped | stem-134 | 16s 3’M | CUA | 888 | |
| rpsH@6 | 988,987,1218 | flipped | stem-134 | 16s 3’M | CUA | 888 | |
| rpsH@7 | 986,1219,1220 | normal | stem-124 | 16s 3’M | UAG | 888 | |
| rpsH@8 | 985,1221,984 | flipped | stem-124 | 16s 3’M | CGC | 888 | |
| rpsH@9 | exit | mid-pond-exit | 16s 3’M | CUA/AG | xX- | ||
| ————— | |||||||
| rpsE@1 | 1040,1000,999 | flipped | stem-124 | 16s 3’M | UAC | 888 | rpsH@1 |
| rpsE@2 | 998,1043,1042 | normal | stem-124 | 16s 3’M | CGA | 888 | rpsH@2 |
| rpsE@3 | 1041 | pond-cross-2x | 16s 3’M | CUG/AA | 8XX | ||
| rpsE@4 | bay-cross-3x | 16s 3’M | UAG/ACA | Xww | |||
| rpsE@5 | 980,981,982 | normal | loop | 16s 3’M | CUU | 888 | |
| rpsE@6 | bay-cross-3x | 16s 3’M | UUU | — | rplD@6 | ||
| rpsE@7 | 993,992,991 | flipped | loop | 16s 3’M | GUU | 888 | rplH@4 |
| rpsE@8 | 990,1215,1216 | normal | stem-124 | 16s 3’M | CGA | 888 | rplH@5, rplD@9 |
| rpsE@9 | 1217,1218 | normal | stem-13x | 16s 3’M | CCG | 88- | rplH@6, rplD@8 |
| rpsE@10 | exit | side-exit | 16s 3’M | CUU | — | ||
| ————— | |||||||
| rplC@1 | 2440,2439,2438 | flipped | loop-stem | 23s V | UAC | 888 | rplD@65 |
| rplC@2 | 2441,2070,2071 | stem-124 | 23s V | UAA | 888 | rplD@65 | |
| rplC@3 | 2072,2073,2435 | stem-136 | 23s V | CCA | 888 | rplD@64 | |
| rplC@4 | 2434,2433,2243 | pond-cross | 23s V | AAU | 888 | ||
| rplC@5 | 2078,2077,2242 | stem-132 | 23s V | CAG | 888 | ||
| rplC@6 | 2247,2248 | pond-cross | 23s V | CCA | x88 | ||
| rplC@7 | 2245,2244,2243 | loop | 23s V | UUU | 888 | ||
| rplC@8 | 2074,2075,2076 | loop | 23s V | UUU | 888 | rplD@63 | |
| ————— | |||||||
| rplC@205 | 2249,2250,2251 | loop-stem | 23s V | GGU | 888 | ||
| rplC@206 | 2248,2256,2247 | stem-123 | 23s V | CGA | 888 | ||
| rplC@207 | 2258,2247,2248 | stem-135 | 23s V | CAC | 888 | rplC@6 | |
| rplC@208 | 2257,2259,2260 | cross-stem | 23s V | UUC | 888 | ||
| rplC@209(end) | 2261,2279,2260 | cross-stem | 23s V | CGC | 888 | ||
| +————— | —————— | ———— | ——————– | ————- | ———- | ———– | ——————-+ |
H Sapiens
Ribosomal proteins S4 and L4 found in a similar shaped region as rpsD/rplD - the genes for S4/L4 in E Coli:
| Protein@Codon | rRNA Indices | Type(WIP) | Match Pattern | rRNA Region | rRNA code | Flow | Partners |
|---|---|---|---|---|---|---|---|
| rps4x@1 | 1043,1080,1044 | stem-123 | 18s | UAC | 888 | ||
| rps4x@2 | 1078,1079,1080 | stem-135 | 18s | CGA | 888 | ||
| rps4x@3 | 1042,1081,1041 | stem-123 | 18s | GCA | 888 | ||
| rps4x@4 | stem-to-bay | 18s | CCA | ||||
| rps4x@5 | 1046,1048,1049 | stem-to-loop-jump | 18s | GGG | 888 | ||
| rps4x@6 | 1050,1051,1052 | loop-to-one-stem | 18s | UUC | 888 | ||
| rps4x@7 | 1075,1076,1077 | loop-to-stem | 18s | UUC | 888 | ||
| rps4x@8 | 1046,1076,1047 | stem-143 | 18s | GUA | 888 | ||
| rps4x@9 | 1074,1073,1052 | loop-stem-twist | 18s | GAC | 888 | ||
| rps4x@10 | 1075,1076,1077 | loop-to-stem | 18s | UUC | 888 | rps4x@7 | |
| rps4x@11 | 1046,1077,1078 | stem-124 | 18s | GCC | 888 | ||
| rps4x@12 | 1044,1080,1081 | stem-126 | 18s | CAC | 888 | ||
| rps4x@13 | 1082,1042,1043 | loop-stem-twist | 18s | CGU | 888 | ||
| rps4x@14 | 1044,1079,1080 | stem-124 | 18s | CGA | 888 | ||
| rps4x@15 | 1042 | exit | exit-stem-base | 18s | GGU | 8– | |
| ————— | |||||||
| rpl4@1 | 1043,1080,1044 | stem-123 | 18s | UAC | 888 | rps4x@1 | |
| rpl4@2 | 1078,1046,1077 | stem-143 | 18s | CGC | 888 | ||
| rpl4@3 | 1078,1046,1077 | stem-143 | 18s | ACA | 88- | ||
| rpl4@4 | 1078,1079,1080 | stem-135 | 18s | CGA | 888 | ||
| rpl4@5 | 1079,1078,1046 | stem-136 | 18s | GCG | 888 | ||
| rpl4@6 | 1048,1049,1051 | loop-with-skip | 18s | GGU | 888 | ||
| rpl4@7 | 1076,1075,1052 | loop-stem-flip | 18s | GAC | 888 | ||
| rpl4@8 | 1077,1047,1078 | loop-cross-stem | 18s | UAU | 888 | ||
| rpl4@9 | 1047,1046,1078 | stem-136 | 18s | AGC | 888 | ||
| rpl4@10 | 1044,1080,1081 | stem-146 | 18s | CAC | 888 | ||
| rpl4@11 | 1083,1084,1042 | loop-stem-cross | 18s | AUG | 888 | ||
| rpl4@12 | 1041 | exit | bay-exit | 18s | AGG | 8– | |
| ————— | |||||||
| rpl4@283 | 1119,1120,1121 | loop | 18s | UUC | 888 | ||
| rpl4@284 | 1125,1124,1123 | loop-to-stem | 18s | UAC | 888 | ||
| rpl4@285 | 1119,1124,1118 | stem-123 | 18s | UAA | 888 | ||
| rpl4@286 | 1117,1116,1127 | stem-132 | 18s | UUA | 888 | ||
| rpl4@287 | 1117,1126,1125 | stem-124 | 18s | UGU | 888 | ||
| rpl4@288 | 1121,1119,1124 | fold-over-tip | 18s | CUA | 888 | ||
| rpl4@289 | 1118 | flexing-fold | 18s | GAA | -8- | ||
| rpl4@290 | 1116,1114,1115 | stem-bump-flip | 18s | UCG | 888 | ||
| rpl4@291 | 1117,1128,1116 | weird-stem-bend | 18s | UCU | 888 | ||
| rpl4@292 | 1125,1117,1126 | stem-123 | 18s | UAG | 888 | ||
| rpl4@293 | 1118,1124,1123 | stem-to-loop | 18s | AAC | 888 | ||
| rpl4@294 | 1119,1120 | loop-exit | 18s | UUU | 88- | ||
| +————— | —————— | ———— | ——————– | ————- | ———- | ———– | ——————-+ |
Alpha Fold Images - E Coli - rplD
For the three sites, notice that all matches occur in strand, or just at the start of a ribbon section. The shapes roughly match up though Alpha Fold’s accuracy is limited in precision by the resolution of crystalography techniques used to train it.
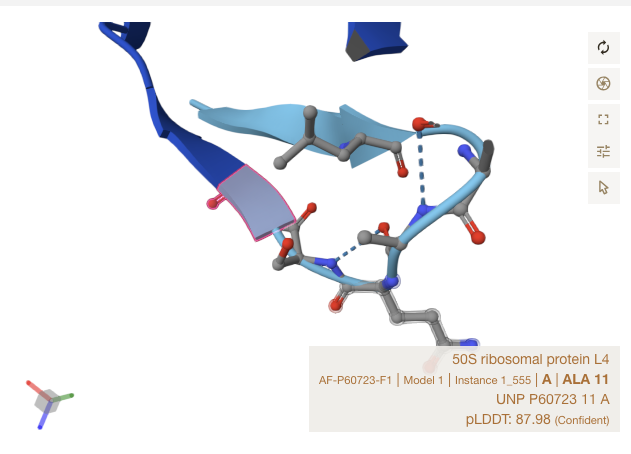
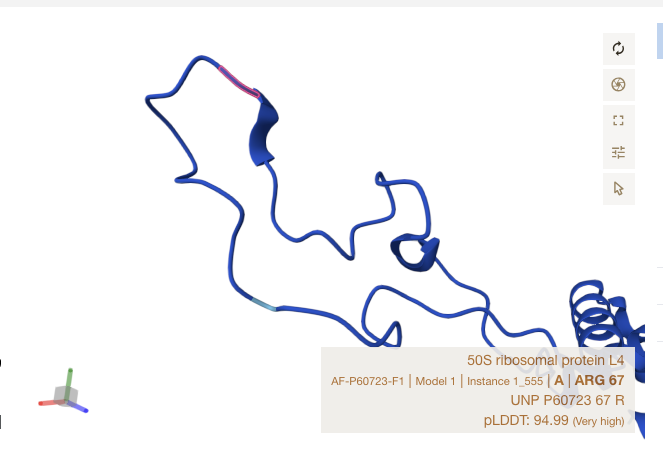
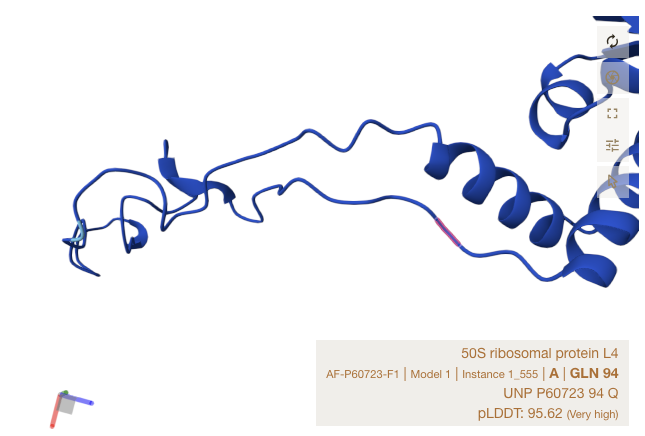
Conclusions
There is too much pattern here for this to be random. These patterns show how the ribosome proteins tie together the two rRNA skeletons, how the flowing rhythmic behavior is formed using patterned bonds that are designed to flex and contract. This is how each tRNA + amino acid gets drawn in, and advanced from stage to stage. I’m not an expert in this field but I believe this is consistent with existing science and adds a layer of understanding.
My personal takeaways include:
- The bonding pattern for amino acids in a protein chain are influenced by the codon pattern.
- An amino acid’s codon pattern affects its bonding so it will bond with an opposite pattern using figure 8 attraction form opposite orbital signature patterns.
- If dark matter orbital signatures are the forces behind covalent bonds, they explain why codons can transmit patterns: dark orbital signatures have state, memory and programmable with fluid flow.
- Cellular dynamics are controlled by fluid flow systems - ordered, minimizing turbulence, not entropy and chaos.
- The tRNA acts like a noise-cancelling amplifier that transfers the codon’s orbital signature to its amino acid.
- When the tRNA attaches to it’s corresponding mRNA, the energy from the ATP is flows into the rRNA, expanding the skeleton, the proteins manage the timing and contraction phase after that energy stabilizes. The spacing, and timing patterns are built into the loops of the rRNA and held together by the proteins of the ribosome. The exact form, shape, stretchiness, contraction pattern is complex but you can see it from the fluid interactions of these connections and interconnections.
- Lack of flow in this system will stand out… cause turbulence. So incorrect patterns, fluid flow, disharmony will shuts it down. Fluid flow drives the whole process. For example, the leader and follower sections have very different flow patterns so snRNPs that help cut out non-coding sections could be flow control devices.
- ATP’s energy is released but conserved. Dark matter fluid is designed to preserve it’s spinning nature, minimize disruption, preserve the spinning, finding spinning/counter-spinning flows that work at a lower energy state, i.e. less turbulence. Otherwise, it spins indefinitely (or for our universe’s lifecycle). To eliminate the turbulence with spinning and counter-spinning boundary layers. Cells are built on that same paradigm.
- Ribosomal proteins maintain the shape and flow, ensure the right contact points are met. The rRNA is the main fluid flow channeller. It’s shape can either be seen as a fluid flow system, with circular eddies forming in the ponds and canyons in the bays, or as a landing strip for arriving proteins, guided in on fluid flow till they find the right stem connections. So the shape of the rRNA is both defining how it gets assembled, how it expands as the next tRNA arrives, how proteins help it contract with the right timing and shape.
This discovery opens up a whole new level of understanding how things work.
Next Steps
Code these patterns into the genetic analyzer so it can detect back-to-back 2 codon in a row sequences.
Find all RNA that lives in each region of each cell and automate the process of mapping RNA to Proteins. How many proteins use RNA as a skeleton or skin?
Code the genetic analyzer to find 2+ long bonding patterns between proteins using it’s partners mechanism.
More Info:
The genetic analyzer - a tool that analyzes proteins preserving the codon information.
Fluid DNA - More info on how the process works with fluid dynamics.